| The law of atmospheres, also known as the barometric law, states that the pressure n(y) as a function of height y varies as: | |
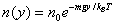 | |
According to the ideal gas law, a gas of N particles in the thermal equilibrium obeys the relationship PV = NkBT. It is convenient to rewrite this equation in terms of the number of particles per unit volume of gas, nV = N/V. This quantity is important because it can vary from one point to another. In fact, our goal is to determine how nV changes in our atmosphere. We can express the ideal gas law in terms of nV as P = nVkBT. Thus, if the number density nV is known, we can find the pressure and vice versa. | |
| The pressure in the atmosphere decreases as the altitude increases because a given layer of air has to support the weight of the air above it — the greater the altitude, the less the weight of the air above that layer and the lower the pressure. | |
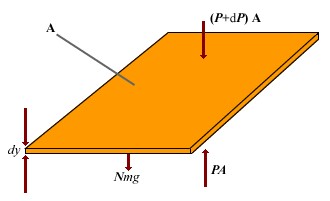 | |
To determine the variation in pressure with altitude, consider an atmospheric layer of thickness dy and the cross-sectional area A. Because the air is in static equilibrium, the upward force on the bottom of this layer, PA, must exceed the downward force on the top of the layer, (P + dP)A, by an amount equal to the weight of gas in this thin layer. If the mass of gas molecule in the layer is m, and the area a total of N molecules in the layer, then the weight of the layer is w = mgN = mgnVAdy. Thus A - (P + dP)A = mgnVAdy, Which reduces to dP = - mgnVdy Because P = nVkBT, and T is assumed to remain constant, therefore dP = nVkBT dnV. | |
| Substituting this into the above expression for dP and rearranging gives | |
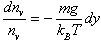 | |
| Integrating this expression, we find | |
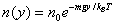 | |
| Boltzmann distribution law | |
Boltzmann distribution law is important in describing the statistical mechanics of a large number of particles. It states that the probability of finding the particles in a particular energy state varies exponentially as the negative of the energy divided by kBT. All the particles would fall into the lowest energy level, except that the thermal energy kBT tends to excite the particles to higher energy levels. | |
Distribution of particles in space is | |
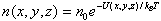 | |
| Where n0 is the number of particles where U = 0 This king of distribution applies to any energy the particles have, such as kinetic energy. In general, the relative number of particles having energy E is | |
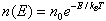 | |
Law of Atmospheres and Boltzmann Law
Labels:
physics topics
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment